What Do Atoms Form When They Share Electron Pairs
What Do Atoms Form When They Share Electron Pairs - Let’s consider both types of bonds in detail. Web if a pair of atoms has a slight positive charge, sharing electrons lets them balance their charge and a bond is formed. The electrons involved are in the outer shells of the atoms. In a double bond, atoms share two pairs of electrons. So those electrons belong to both of those atoms. Web study with quizlet and memorize flashcards containing terms like 4 elements that make up 96% of living matter, what do atoms form when they share electron pairs, explain most specifically the attraction of water molecules to one another and more.
Let’s consider both types of bonds in detail. Web a single shared pair of electrons is called a single bond. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. The six in the lone pairs and the two in the single bond.
A Covalent Bond Forms When The Bonded Atoms Have A Lower Total Energy Than That Of Widely Separated Atoms.
Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. In a double bond, atoms share two pairs of electrons. Web each atom donates a single electron to the electron pair which is shared. More than a century ago, in 1916, gilbert lewis identified the chemical bond with a pair of shared electrons in an epochal publication titled the atom and the molecule 1.
Because Most Filled Electron Shells Have Eight Electrons In Them, Chemists Called This Tendency The Octet Rule.
If the atoms are identical, like two hydrogens forming \ce {h_ {2 (g)}} hx 2(g), then the bond is purely covalent. They also fill the core levels of an atom. What is the difference between covalent and ionic bonding? Covalent bonding occurs between nonmetallic atoms with similar electronegativities.
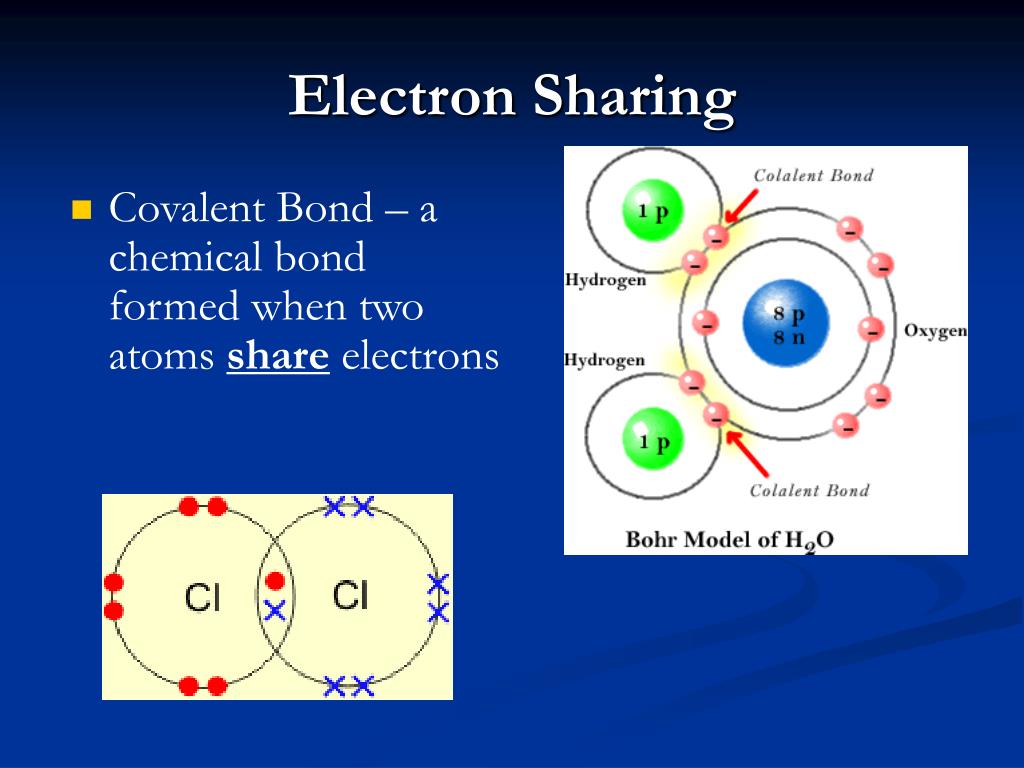
Covalent Bond Is A Chemical Bond That Involves Sharing Of Electron Pairs Between Atoms.
Web a single shared pair of electrons is called a single bond. In a single covalent bond, two atoms share one pair of electrons. When two atoms form a covalent bond, they share their valence electrons in order to achieve an octet (8 valence electrons), except for hydrogen which bonds to achieve a duet (2 valence electrons). Covalent bonding makes the atoms stable.
Double Bonds Or Triple Bonds Between Atoms May Be Necessary To Properly Illustrate The Bonding In Some Molecules.
The binding arises from the electrostatic attraction of their nuclei for the same electrons. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. A covalent bond is formed when two atoms share electron pairs. So those electrons belong to both of those atoms.
If the atoms are identical, like two hydrogens forming \ce {h_ {2 (g)}} hx 2(g), then the bond is purely covalent. In covalent bonding electrons are shared between two atoms. Lewis electron dot diagrams can be drawn to illustrate covalent bond formation. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. What is the difference between covalent and ionic bonding?

![[ANSWERED] What do atoms form when they equally share electron](https://i2.wp.com/media.kunduz.com/media/sug-question-candidate/20220511034952883386-4536923.jpg?h=512)