Isotopes Worksheet Answers
Isotopes Worksheet Answers - 6 protons & 6 neutrons e. Makes an effective homework or revision resource including a challenge question to stretch higher ability students. 12c 6 13c 6 14c 6. Web isotope practice worksheet 1. How many protons and neutrons are in the first isotope? What do all isotopes of an element have in common?
A short worksheet to introduce or revise isotopes. Web isotopes worksheets and full answers. Web atoms of the same element with different numbers of neutrons are called isotopes. Web isotopes practice set oe aa — 1. The average mass of the balls is given by:
Which Isotope Of Lead Is Likely To Be The Most Abundant?
Here are three isotopes of an element: Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Protons and neutrons are in the nucleus. How many protons and neutrons are in the first.
Calculate The Average Atomic Mass Of Chlorine If Its Isotopes And % Abundances Are As Follows.
If we know the number mass number and the atomic number, we can calculate the number of neutrons in the atom using: The average mass of the balls is given by: The average atomic mass of a lead atom is 207.2 amu. Web isotopes of an element have the same atomic number (z) but have different mass numbers (a), because they have different numbers of neutrons.
5 Protons, 6 Neutrons, 5 Electrons.
Here are three isotopes of an element: It includes a series of questions of increasing challenge, with answers and extra supporting videos available at the link on the bottom of each page or via the qr code. Web in a sample of e there are two isotopes. Web ask the class to read through the remaining sections on the provided worksheet, and answer the questions.
12C 6 13C 6 14C 6.
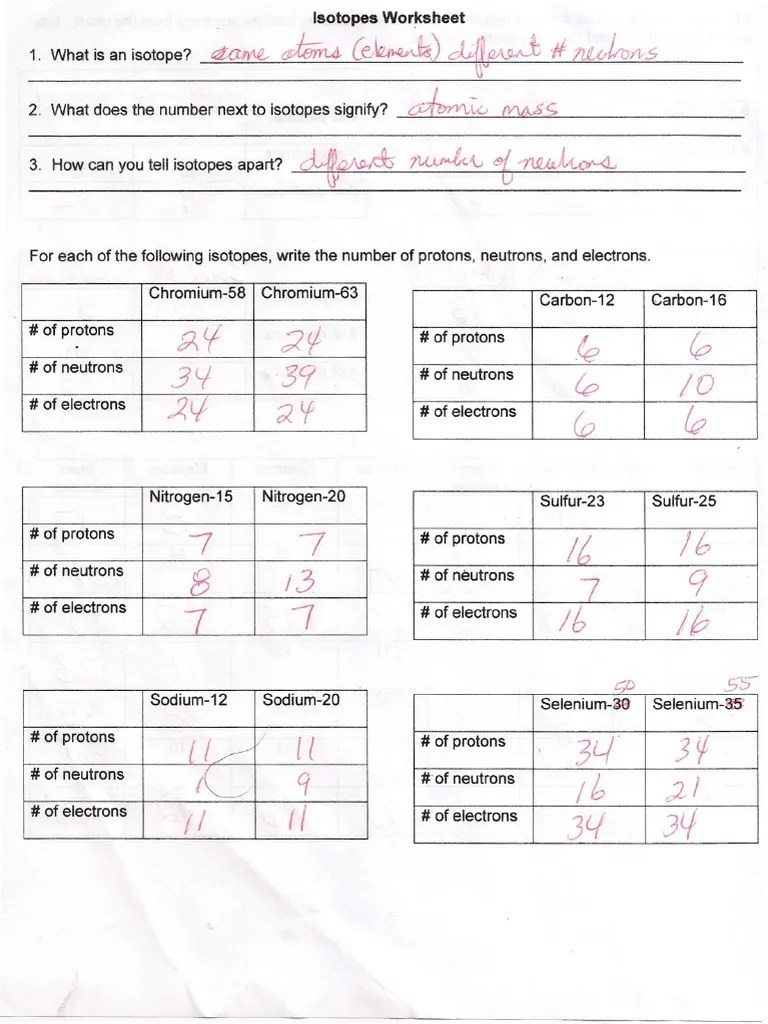
Web for each isotope shown, give the number of protons, neutrons, and electrons. Web isotope practice worksheet 1. Imagine you have 90 balls with mass 200 g, and 10 balls with mass 300 g. Full written answers and a video explanation for this worksheet is also available.
The numbers 12, 13, and 14 refer to the mass number d. Web for each isotope shown, give the number of protons, neutrons, and electrons. For each of the following isotopes, write the number of protons, neutrons, and electrons. Web the relative atomic mass (ar) of atoms is the average mass of all the different isotopes of an element (taking into account the amount of each isotope) on a scale where 12c atoms have a mass of exactly 12. Web atoms of the same element with different numbers of neutrons are called isotopes.